null
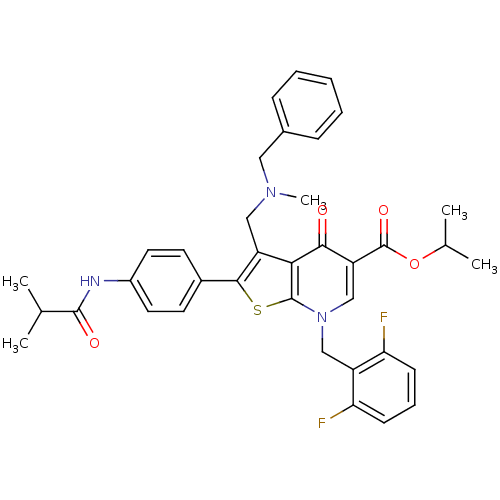
SMILES CC(C)OC(=O)c1cn(Cc2c(F)cccc2F)c2sc(c(CN(C)Cc3ccccc3)c2c1=O)-c1ccc(NC(=O)C(C)C)cc1
InChI Key InChIKey=RANJJVIMTOIWIN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50067485
Found 8 hits for monomerid = 50067485
TargetGonadotropin-releasing hormone receptor(Homo sapiens (Human))
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Binding affinity towards human gonadotropin-releasing hormone receptorMore data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Binding affinity of the compound was determined towards rat Gonadotropin-releasing hormone receptorMore data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Binding affinity of the compound against gonadotropin-releasing hormone receptor of ratMore data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Ability of compound to inhibit [I125-Tyr5,DLeu6,NMeLeu7,Pro9-NEt]GnRH agonist binding to the rat Gonadotropin-releasing hormone receptor was evaluate...More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Homo sapiens (Human))
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Ability of compound to inhibit [I125-Tyr5,DLeu6,NMeLeu7,Pro9-NEt]GnRH agonist binding to the cloned human Gonadotropin-releasing hormone receptor was...More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of Rat gonadotropin-releasing hormone receptor(moderate affinity)More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Homo sapiens (Human))
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of Human gonadotropin-releasing hormone receptorMore data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Homo sapiens (Human))
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Neurocrine Biosciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory activity against human GnRH receptor of the compound using des-Gly10[125I-Tyr5, D-Leu, NMeLeu, Pro-NEt]GnRH radioligandMore data for this Ligand-Target Pair