null
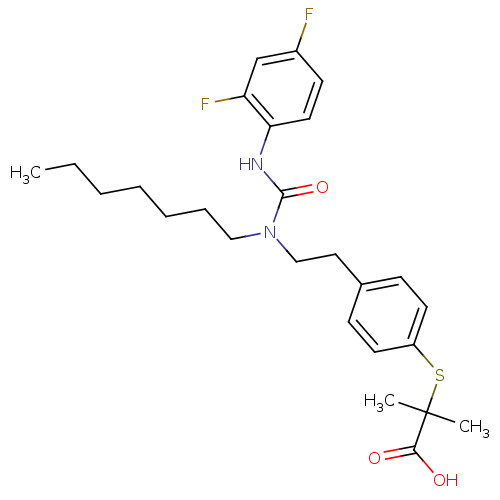
SMILES CCCCCCCN(CCc1ccc(SC(C)(C)C(O)=O)cc1)C(=O)Nc1ccc(F)cc1F
InChI Key InChIKey=KYQNYMXQHLMADB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 28799
Found 4 hits for monomerid = 28799
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Division of Eli Lilly & Company
Curated by ChEMBL
Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibitory activity against human Peroxisome proliferator activated receptor alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Division of Eli Lilly & Company
Curated by ChEMBL
Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataEC50: 1.90E+3nMAssay Description:Cotransfection activity of compound against human Peroxisome proliferator activated receptor gamma was determinedMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Division of Eli Lilly & Company
Curated by ChEMBL
Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 550nMAssay Description:Inhibitory activity of compound against the binding of human Peroxisome proliferator activated receptor gamma was determinedMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Division of Eli Lilly & Company
Curated by ChEMBL
Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataEC50: 340nMAssay Description:Cotransfection activity of compound against human Peroxisome proliferator activated receptor alpha was determinedMore data for this Ligand-Target Pair