null
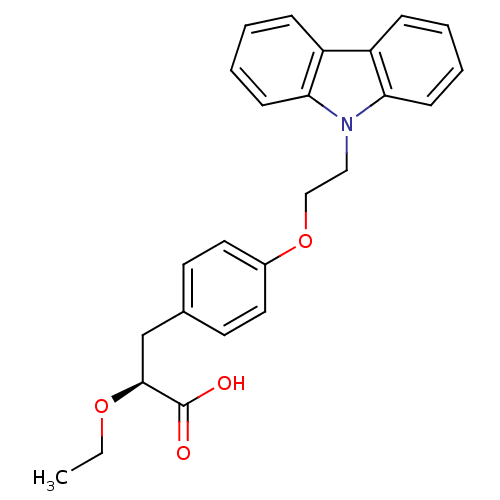
SMILES CCO[C@@H](Cc1ccc(OCCn2c3ccccc3c3ccccc23)cc1)C(O)=O
InChI Key InChIKey=WUZIMDSVRIBNNI-DEOSSOPVSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50109547
Found 2 hits for monomerid = 50109547
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: 170nMAssay Description:Effective concentration against human peroxisome proliferator activated receptor gamma in Gal4 transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataEC50: 360nMAssay Description:Effective concentration against human peroxisome proliferator activated receptor alpha in Gal4 transactivation assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)