null
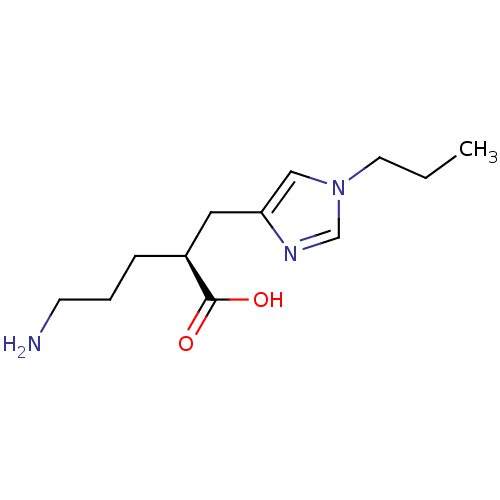
SMILES CCCn1cnc(C[C@H](CCCN)C(O)=O)c1
InChI Key InChIKey=OTDGPKRCQXSTPV-JTQLQIEISA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50226610
Found 4 hits for monomerid = 50226610
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Inhibition of human TAFIaMore data for this Ligand-Target Pair
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 16nMAssay Description:Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 minsMore data for this Ligand-Target Pair
TargetCarboxypeptidase B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 206nMAssay Description:Inhibition of human pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
Affinity DataKi: 206nMAssay Description:Inhibition of porcine pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)