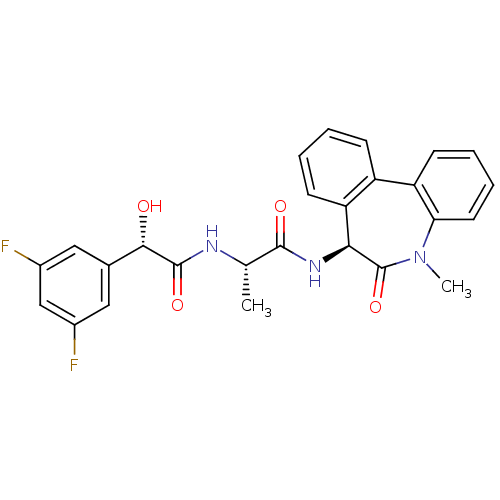
null
SMILES C[C@H](NC(=O)[C@@H](O)c1cc(F)cc(F)c1)C(=O)N[C@H]1c2ccccc2-c2ccccc2N(C)C1=O
InChI Key InChIKey=ULSSJYNJIZWPSB-CVRXJBIPSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50241259
Found 2 hits for monomerid = 50241259
Affinity DataEC50: 0.114nMAssay Description:Reduction of human wild type PS1-induced amyloid beta40 level in CHO cells overexpressing human APP751 after 24 hrs by liquid phase electrochemilumin...More data for this Ligand-Target Pair
Affinity DataEC50: 0.135nMAssay Description:Reduction of human wild type PS1-induced amyloid beta42 level in CHO cells overexpressing human APP751 after 24 hrs by liquid phase electrochemilumin...More data for this Ligand-Target Pair