null
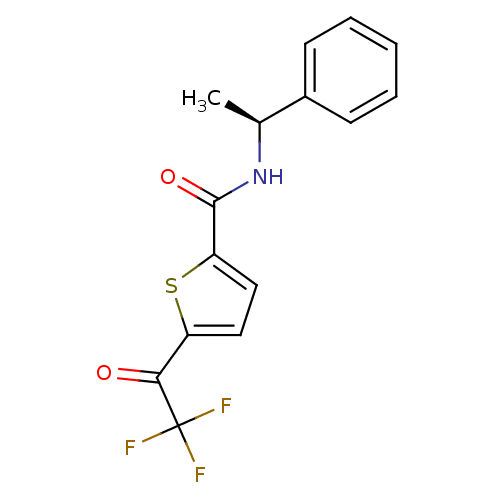
SMILES C[C@H](NC(=O)c1ccc(s1)C(=O)C(F)(F)F)c1ccccc1
InChI Key InChIKey=DPKHRWMGXRDLLO-VIFPVBQESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 25159
Found 4 hits for monomerid = 25159
TargetHistone deacetylase 6(Homo sapiens (Human))
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibition of HDAC6 (unknown origin)More data for this Ligand-Target Pair
TargetHistone deacetylase 1(Homo sapiens (Human))
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
Affinity DataIC50: 5.30E+3nMAssay Description:Inhibition of HDAC1 (unknown origin)More data for this Ligand-Target Pair
TargetHistone deacetylase 3(Homo sapiens (Human))
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
Affinity DataIC50: 9.40E+3nMAssay Description:Inhibition of HDAC3 (unknown origin)More data for this Ligand-Target Pair
TargetHistone deacetylase 4(Homo sapiens (Human))
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Inhibition of His-tagged HDAC4 catalytic domain (unknown origin) expressed in Escherichia coliMore data for this Ligand-Target Pair