null
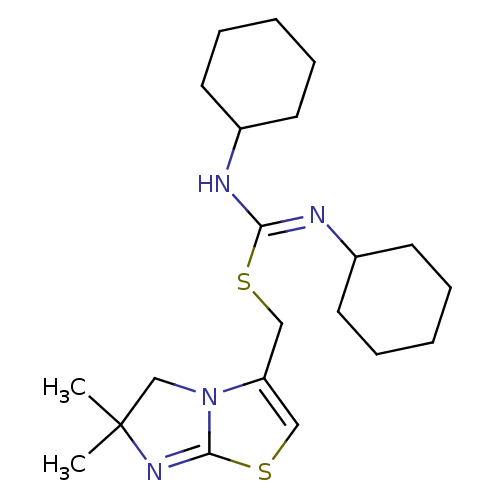
SMILES CC1(C)CN2C(CS\C(NC3CCCCC3)=N/C3CCCCC3)=CSC2=N1
InChI Key InChIKey=OOSUDWRRWZVFEB-UHFFFAOYSA-N
PDB links: 4 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50247128
Found 10 hits for monomerid = 50247128
TargetCytochrome P450 1A2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 5.20E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human recombinant ERG in CHOK1 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.32E+4nMAssay Description:Displacement of [3H]dofetilide from human recombinant ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Displacement of [125I]CXCL12 from CXCR4 in human CEM cellsMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Rattus norvegicus (Rat))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Displacement of [125I]CXCL12 from CXCR4 in rat IR983F cellsMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilizationMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)