null
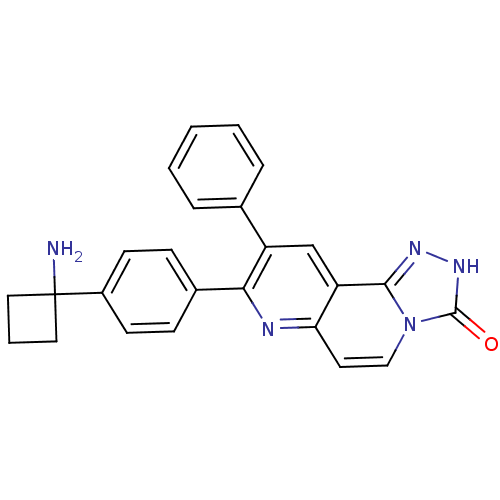
SMILES NC1(CCC1)c1ccc(cc1)-c1nc2ccn3c(n[nH]c3=O)c2cc1-c1ccccc1
InChI Key InChIKey=ULDXWLCXEDXJGE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50313650
Found 3 hits for monomerid = 50313650
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Schering-Plough
Curated by ChEMBL
Schering-Plough
Curated by ChEMBL
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
Schering-Plough
Curated by ChEMBL
Schering-Plough
Curated by ChEMBL
TargetRAC-gamma serine/threonine-protein kinase(Homo sapiens (Human))
Schering-Plough
Curated by ChEMBL
Schering-Plough
Curated by ChEMBL