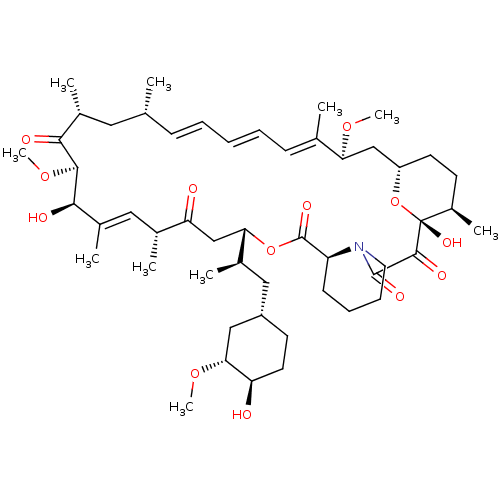
null
SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O
InChI Key InChIKey=QFJCIRLUMZQUOT-HPLJOQBZSA-N
PDB links: 23 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 36609
Found 1 hit for monomerid = 36609
TargetEukaryotic translation initiation factor 4E(Homo sapiens (Human))
University of Toronto
Curated by ChEMBL
University of Toronto
Curated by ChEMBL
Affinity DataKd: 0.5nMAssay Description:Binding affinity to purified elF4EMore data for this Ligand-Target Pair