null
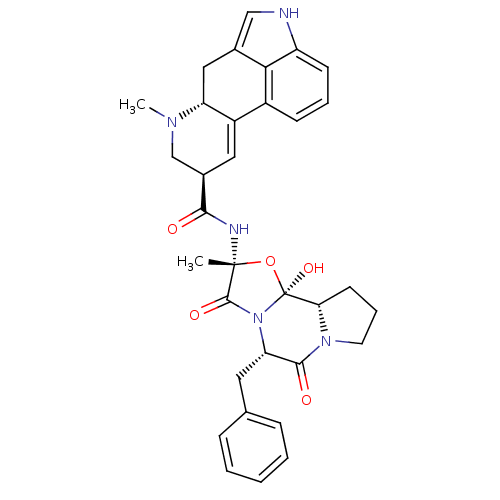
SMILES CN1C[C@@H](C=C2[C@H]1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)[C@@H]3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O
InChI Key InChIKey=XCGSFFUVFURLIX-VFGNJEKYSA-N
PDB links: 6 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50027065
Found 2 hits for monomerid = 50027065
Affinity DataKi: 2.10nMAssay Description:Displacement of [3H]carboxytryptamine from recombinant human 5-HT1B receptor expressed in HEK cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Displacement of [3H]GR125743 from recombinant human 5-HT1D receptor expressed in HEK cells by scintillation countingMore data for this Ligand-Target Pair