null
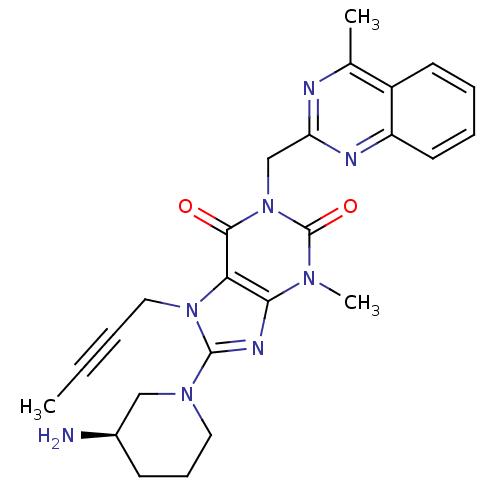
SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1
InChI Key InChIKey=LTXREWYXXSTFRX-QGZVFWFLSA-N
PDB links: 2 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50228403
Found 3 hits for monomerid = 50228403
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Argenta Discovery Ltd.
Curated by ChEMBL
Argenta Discovery Ltd.
Curated by ChEMBL
Affinity DataIC50: 295nMAssay Description:Inhibition of M1 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Argenta Discovery Ltd.
Curated by ChEMBL
Argenta Discovery Ltd.
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG by dofetilide binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus systemMore data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2SF2WN8PubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2SF2WN8PubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)