null
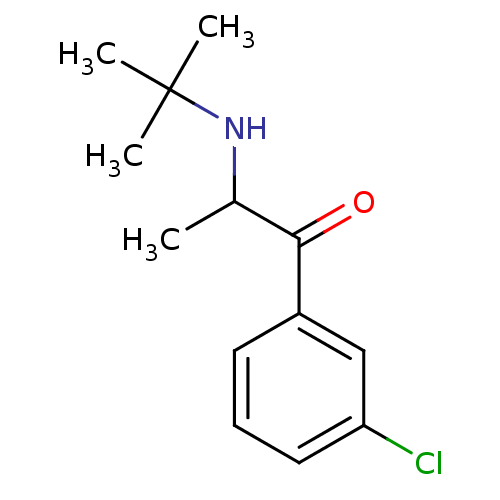
SMILES CC(NC(C)(C)C)C(=O)c1cccc(Cl)c1
InChI Key InChIKey=SNPPWIUOZRMYNY-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 50048392
Found 13 hits for monomerid = 50048392
Affinity DataIC50: 1.23E+3nMAssay Description:Compound was tested for its ability to inhibit the neurotransmitter dopamine-DA reuptake system using [3H]dopamine as radioligandMore data for this Ligand-Target Pair