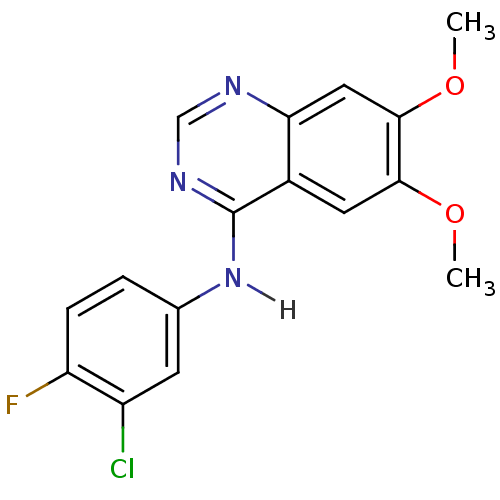
null
SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OC
InChI Key InChIKey=VOPNWXZDJKCCRE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 4626
Found 3 hits for monomerid = 4626
Affinity DataIC50: 340nMAssay Description:Compound was evaluated for its concentration required to inhibit the porcine kidney F16BPaseMore data for this Ligand-Target Pair
Affinity DataIC50: 290nMAssay Description:Concentration required to inhibit the human liver recombinant fructose-1,6-bisphosphatase.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Compound was evaluated for its concentration required to inhibit the rat liver F16BPaseMore data for this Ligand-Target Pair