null
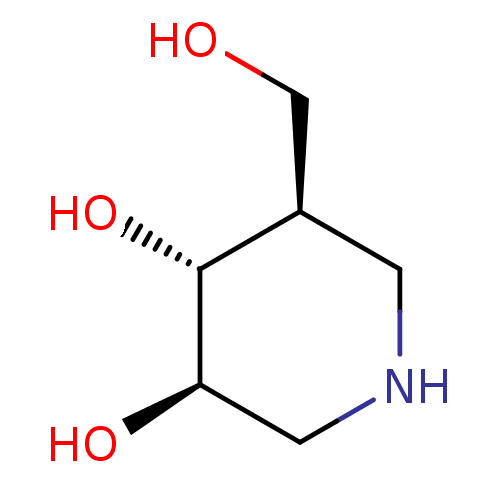
SMILES OC[C@H]1CNC[C@@H](O)[C@@H]1O
InChI Key InChIKey=QPYJXFZUIJOGNX-HSUXUTPPSA-N
PDB links: 26 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50182801
Found 3 hits for monomerid = 50182801
Affinity DataIC50: 60nMAssay Description:Inhibition of human lysosomal beta-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+5nMAssay Description:Inhibition of rabbit muscle amylo-1,6-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibition of liver glycogen phosphorylase AMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)