null
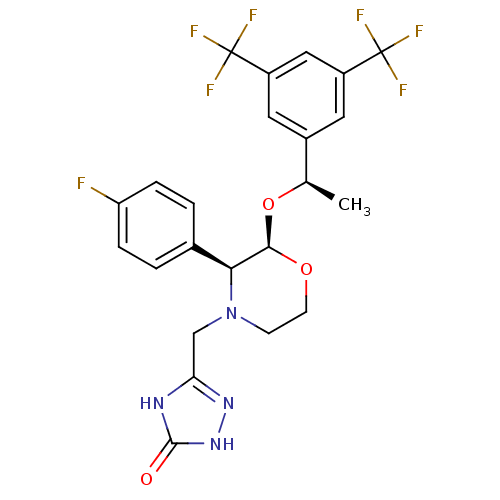
SMILES C[C@@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F
InChI Key InChIKey=ATALOFNDEOCMKK-OITMNORJSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50220136
Found 2 hits for monomerid = 50220136
Affinity DataKi: 3nMAssay Description:Displacement of radioligand [3H]SP from wild type human NK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 454nMAssay Description:Displacement of [3H]osanetant from wild type human NK3 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair