null
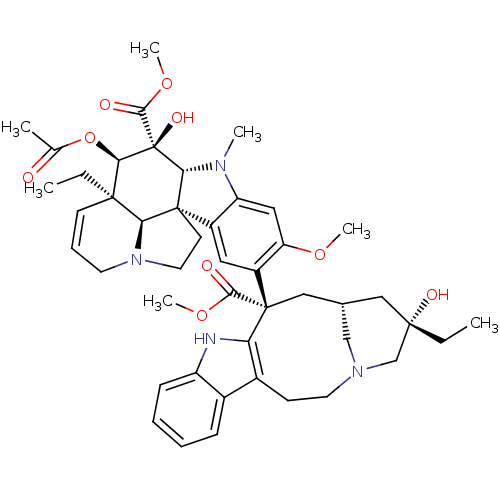
SMILES CC[C@]1(O)C[C@@H]2CN(C1)CCc1c([nH]c3ccccc13)[C@@](C2)(C(=O)OC)c1cc2c(cc1OC)N(C)[C@@H]1[C@]22CCN3CC=C[C@](CC)([C@@H]23)[C@@H](OC(C)=O)[C@]1(O)C(=O)OC
InChI Key InChIKey=JXLYSJRDGCGARV-CFWMRBGOSA-N
PDB links: 7 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50012278
Found 1 hit for monomerid = 50012278
TargetNuclear receptor subfamily 0 group B member 1(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay