null
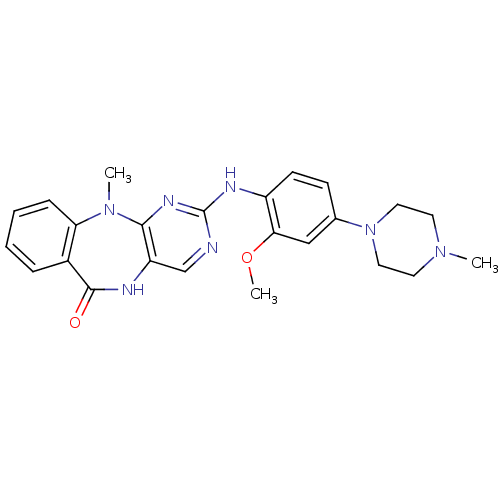
SMILES COc1cc(ccc1Nc1ncc2NC(=O)c3ccccc3N(C)c2n1)N1CCN(C)CC1
InChI Key InChIKey=LGLHCXISMKHLIK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50337126
Found 4 hits for monomerid = 50337126
Affinity DataKd: 15nMAssay Description:In vitro biochemical assays were performed in parallel to determine the most potent tool compound.More data for this Ligand-Target Pair
Affinity DataKd: 2nMAssay Description:Inhibition of ACK1 kinase (unknown origin)More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 44nMAssay Description:Inhibition of tracer 236 binding to recombinant human GST-tagged TNK2 catalytic domain (110 to 476 residues) expressed in baculovirus expression syst...More data for this Ligand-Target Pair