null
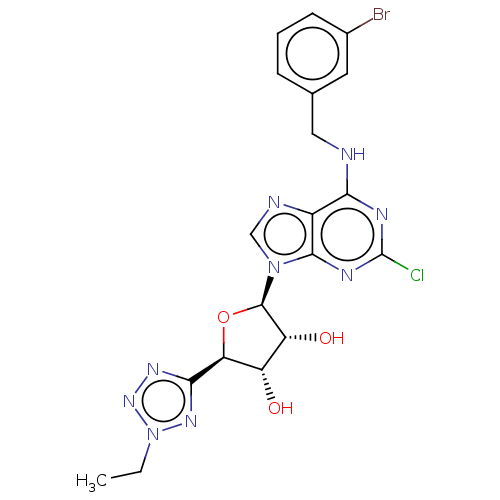
SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(Br)c3)nc(Cl)nc12
InChI Key InChIKey=WTSRCKFFAWSKBH-MOROJQBDSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50266664
Found 2 hits for monomerid = 50266664
Affinity DataKi: 1.80nMAssay Description:Displacement of [3H]CCPA from recombinant human adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 73nMAssay Description:Agonist activity at recombinant human adenosine A1 receptor expressed in CHO cell membranes assessed as inhibition of forskolin-stimulated adenylyl c...More data for this Ligand-Target Pair