null
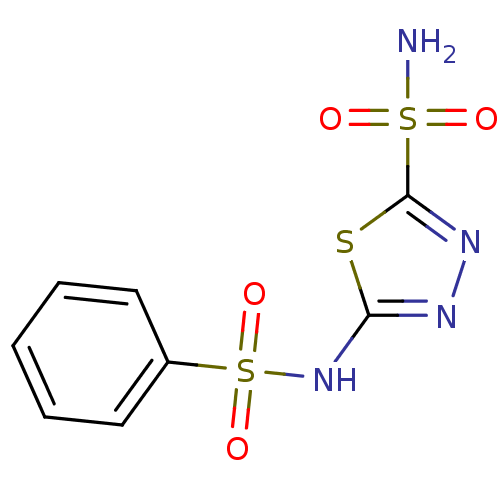
SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccccc2)s1
InChI Key InChIKey=PWDGTQXZLNDOKS-UHFFFAOYSA-N
PDB links: 2 PDB IDs match this monomer. 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 10886
Found 3 hits for monomerid = 10886
Affinity DataKi: 3.5nMAssay Description:Inhibitory constant against catalytic domain of human carbonic anhydrase XIIMore data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 3.80nMAssay Description:Ki value against human carbonic anhydrase XII (hCA XII)More data for this Ligand-Target Pair