null
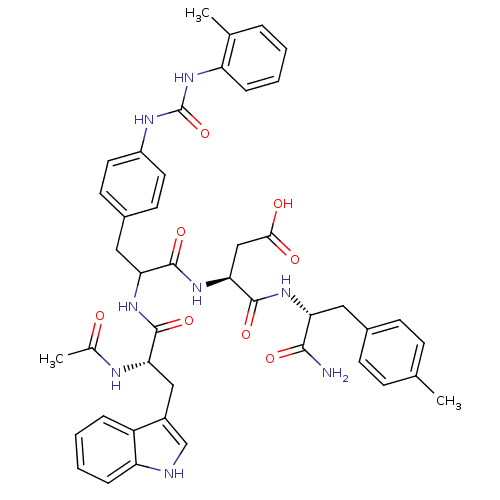
SMILES CC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC(Cc1ccc(NC(=O)Nc2ccccc2C)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](Cc1ccc(C)cc1)C(N)=O
InChI Key InChIKey=KEYXCQSIKSZIRX-NGIIJNKUSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50407325
Found 1 hit for monomerid = 50407325
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Glaxo Research Institute
Curated by ChEMBL
Glaxo Research Institute
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Displacement of CCK-8 from CHO cell membranes expressing human Cholecystokinin type A receptorMore data for this Ligand-Target Pair