null
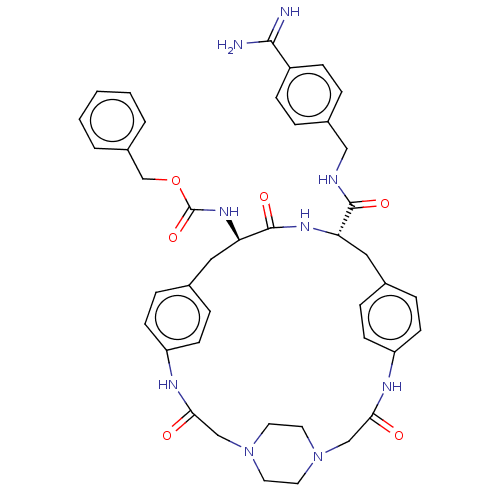
SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NC(=O)OCc1ccccc1
InChI Key InChIKey=VVGWFOCDEJJLPJ-VMYQRNJMSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50532386
Found 2 hits for monomerid = 50532386
Affinity DataKi: 1.04E+5nMAssay Description:Inhibition of human f10a using Mes-DArg-Pro-Arg-AMC as substrate by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.04E+5nMAssay Description:Inhibition of human f10a using Mes-DArg-Pro-Arg-AMC as substrate by Dixon plot analysisMore data for this Ligand-Target Pair