null
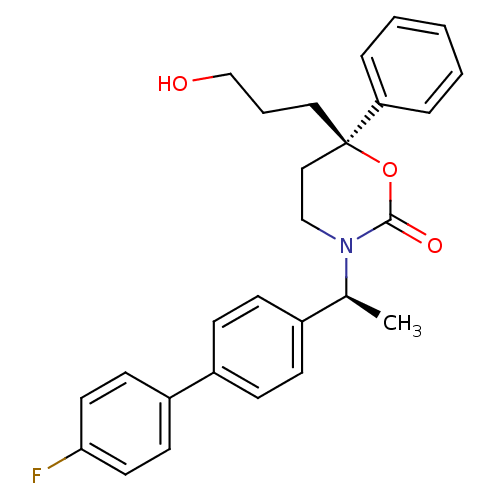
SMILES C[C@H](N1CC[C@@](CCCO)(OC1=O)c1ccccc1)c1ccc(cc1)-c1ccc(F)cc1
InChI Key InChIKey=NRQVLVGDMUNLPF-CCLHPLFOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50353392
Found 2 hits for monomerid = 50353392
TargetCytochrome P450 3A4(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH
US Patent
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH
US Patent
Affinity DataIC50: 7.00E+3nMT: 2°CAssay Description:The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH
US Patent
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH
US Patent
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of human CYP3A4 assessed as rate of [14C]-formaldehyde/formic acid production by liquid scintillation countingMore data for this Ligand-Target Pair