null
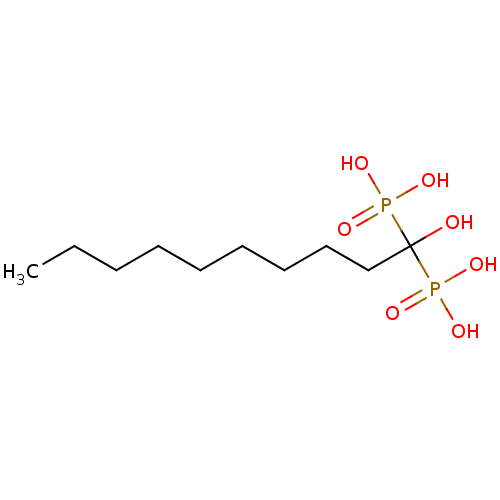
SMILES CCCCCCCCCC(O)(P(O)(O)=O)P(O)(O)=O
InChI Key InChIKey=NSCPCMXKWUFFNL-UHFFFAOYSA-N
PDB links: 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 25269
Found 3 hits for monomerid = 25269
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
University of Illinois at Urbana-Champaign
Curated by ChEMBL
University of Illinois at Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 230nMAssay Description:Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligandMore data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
University of Illinois at Urbana-Champaign
Curated by ChEMBL
University of Illinois at Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibitory activity against farnesyl Pyrophosphate Synthase was determinedMore data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
University of Illinois at Urbana-Champaign
Curated by ChEMBL
University of Illinois at Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 2.34E+3nMAssay Description:Inhibitory activity against farnesyl Pyrophosphate Synthase expressed as #NAME? (M)More data for this Ligand-Target Pair