null
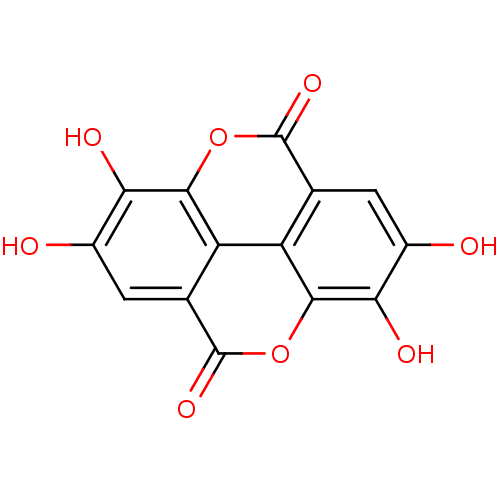
SMILES Oc1cc2c3c(oc(=O)c4cc(O)c(O)c(oc2=O)c34)c1O
InChI Key InChIKey=AFSDNFLWKVMVRB-UHFFFAOYSA-N
PDB links: 3 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 4078
Found 3 hits for monomerid = 4078
Affinity DataEC50: 110nMAssay Description:Agonist activity at GPR35 receptor in human HT-29 cells after 10 mins by dynamic mass redistribution assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.96E+3nMAssay Description:Agonist activity at GPR35 receptor in human U2OS cells coexpressing Gal4-VP16-TEV assessed as beta arrestin translocation after 5 hrs by beta lactmas...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Desensitization of GPR35 receptor in human HT-29 cells assessed as inhibition of zaprinast-induced dynamic mass redistribution after 10 minsMore data for this Ligand-Target Pair