null
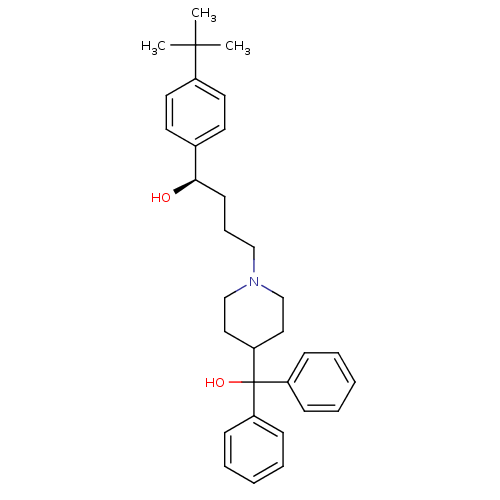
SMILES CC(C)(C)c1ccc(cc1)[C@H](O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1
InChI Key InChIKey=GUGOEEXESWIERI-SSEXGKCCSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 22879
Found 3 hits for monomerid = 22879
Affinity DataKd: 380nMAssay Description:Inhibition of specific binding of [3H]mepyramine to Histamine 1 receptors in guinea-pigMore data for this Ligand-Target Pair