null
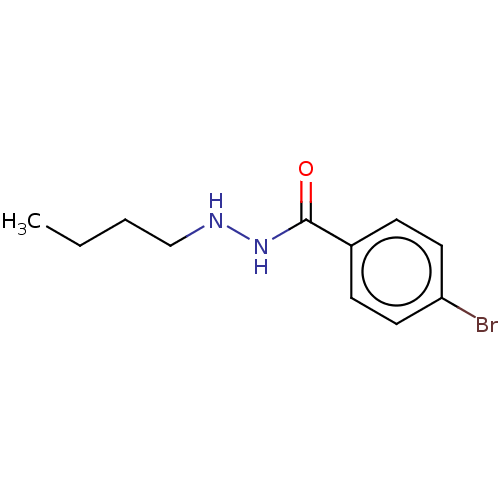
SMILES CCCCNNC(=O)c1ccc(Br)cc1
InChI Key InChIKey=BVQCFCYPFJOOAV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 163619
Found 6 hits for monomerid = 163619
TargetHistone deacetylase 2(Homo sapiens (Human))
University of Florida Research Foundation, Inc.; The Scripps Research Institute
US Patent
University of Florida Research Foundation, Inc.; The Scripps Research Institute
US Patent
Affinity DataIC50: 100nMAssay Description:These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic...More data for this Ligand-Target Pair
TargetHistone deacetylase 2(Homo sapiens (Human))
University of Florida Research Foundation, Inc.; The Scripps Research Institute
US Patent
University of Florida Research Foundation, Inc.; The Scripps Research Institute
US Patent
Affinity DataIC50: 100nMAssay Description:Inhibition of human HDAC2 expressed in Sf9 cells using p53 (379 to 382 residues) (Arg-His-Lys(Ac)-Lys(Ac)) as substrate by fluorimetric assayMore data for this Ligand-Target Pair
TargetHistone deacetylase 2(Homo sapiens (Human))
University of Florida Research Foundation, Inc.; The Scripps Research Institute
US Patent
University of Florida Research Foundation, Inc.; The Scripps Research Institute
US Patent
TargetHistone deacetylase 2(Homo sapiens (Human))
University of Florida Research Foundation, Inc.; The Scripps Research Institute
US Patent
University of Florida Research Foundation, Inc.; The Scripps Research Institute
US Patent
TargetHistone deacetylase 2(Homo sapiens (Human))
University of Florida Research Foundation, Inc.; The Scripps Research Institute
US Patent
University of Florida Research Foundation, Inc.; The Scripps Research Institute
US Patent
TargetHistone deacetylase 2(Homo sapiens (Human))
University of Florida Research Foundation, Inc.; The Scripps Research Institute
US Patent
University of Florida Research Foundation, Inc.; The Scripps Research Institute
US Patent
Affinity DataIC50: 100nMpH: 8.0Assay Description:Activity against HDACs 1 to 11 was assessed by using an acetylated 7-amino-4-methylcoumarin (AMC)-labeled peptide substrate. A substrate based on res...More data for this Ligand-Target Pair