null
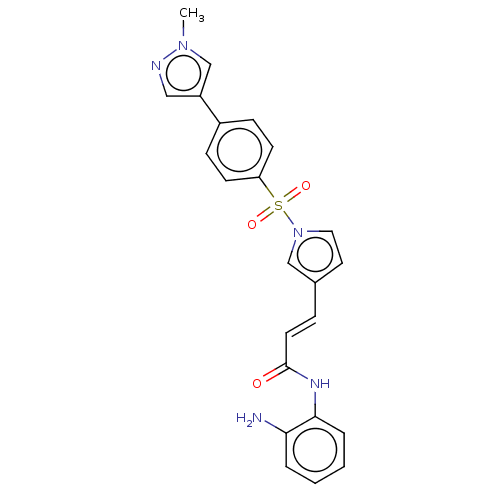
SMILES Cn1cc(cn1)-c1ccc(cc1)S(=O)(=O)n1ccc(\C=C\C(=O)Nc2ccccc2N)c1
InChI Key InChIKey=PRXXYMVLYKJITB-IZZDOVSWSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50470579
Found 4 hits for monomerid = 50470579
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of HDAC2 (unknown origin) using p53 residues (379 to 382)(RHKK(Ac)-AMC) as substrate by fluorescent methodMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails