null
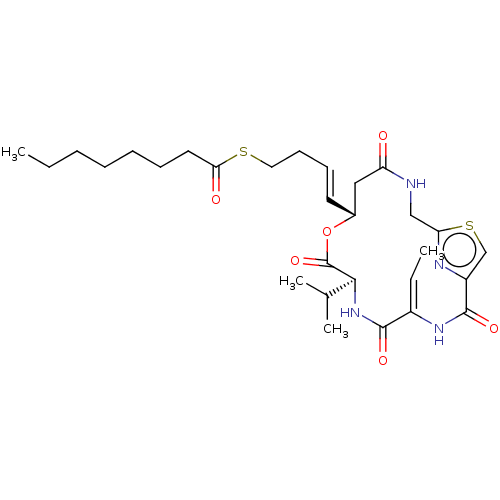
SMILES CCCCCCCC(=O)SCC\C=C\[C@@H]1CC(=O)NCc2nc(cs2)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O1
InChI Key InChIKey=WCZYLKXWWUKEBA-QUDUTUPQSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50123628
Found 2 hits for monomerid = 50123628
TargetHistone deacetylase 8(Homo sapiens (Human))
Guangzhou Institute of Biomedicine and Health
Curated by ChEMBL
Guangzhou Institute of Biomedicine and Health
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant human HDAC8 after 60 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetHistone deacetylase 8(Homo sapiens (Human))
Guangzhou Institute of Biomedicine and Health
Curated by ChEMBL
Guangzhou Institute of Biomedicine and Health
Curated by ChEMBL
Affinity DataIC50: 3.96E+3nMAssay Description:Inhibition of recombinant human full length C-terminal His-tagged HDAC8 expressed in baculovirus infected Sf9 insect cells using fluorometric substra...More data for this Ligand-Target Pair