null
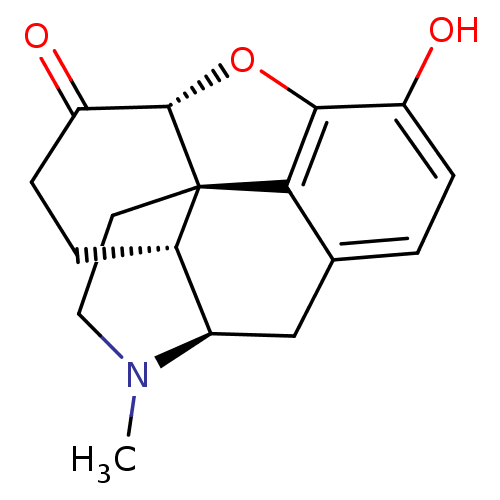
SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3CCC4=O)ccc5O
InChI Key InChIKey=WVLOADHCBXTIJK-YNHQPCIGSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50241341
Found 2 hits for monomerid = 50241341
TargetKappa-type opioid receptor(Homo sapiens (Human))
Rensselaer Polytechnic Institute
Curated by ChEMBL
Rensselaer Polytechnic Institute
Curated by ChEMBL
Affinity DataKi: 2.80nMAssay Description:Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation countingMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
Rensselaer Polytechnic Institute
Curated by ChEMBL
Rensselaer Polytechnic Institute
Curated by ChEMBL
Affinity DataEC50: 11nMAssay Description:Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS bindingMore data for this Ligand-Target Pair