null
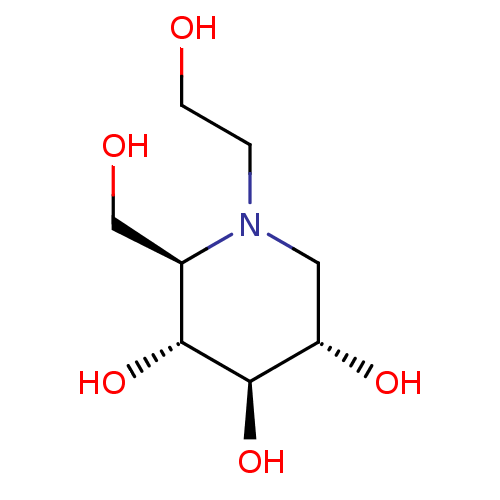
SMILES OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO
InChI Key InChIKey=IBAQFPQHRJAVAV-ULAWRXDQSA-N
PDB links: 4 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50242271
Found 5 hits for monomerid = 50242271
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of human small intestine microsomal maltase using maltose as substrate incubated for 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of maltase by HPLCMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of human intestinal maltase using maltose as substrate incubated for 30 mins and immediately heated for 2 mins by glucose oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.54E+5nMAssay Description:In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)