null
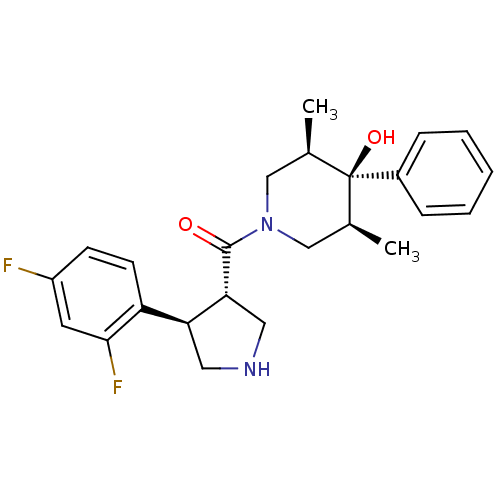
SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@@H]1CNC[C@H]1c1ccc(F)cc1F
InChI Key InChIKey=MXAUIAYYRYRHPY-NGZAGFBYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50315680
Found 2 hits for monomerid = 50315680
TargetMelanocortin receptor 4(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 264nMAssay Description:Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation countingMore data for this Ligand-Target Pair
TargetMelanocortin receptor 4(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataEC50: 462nMAssay Description:Agonist activity at human recombinant MC4 receptor expressed in CHO cells by cAMP responsive beta lactamase reporter gene assayMore data for this Ligand-Target Pair