null
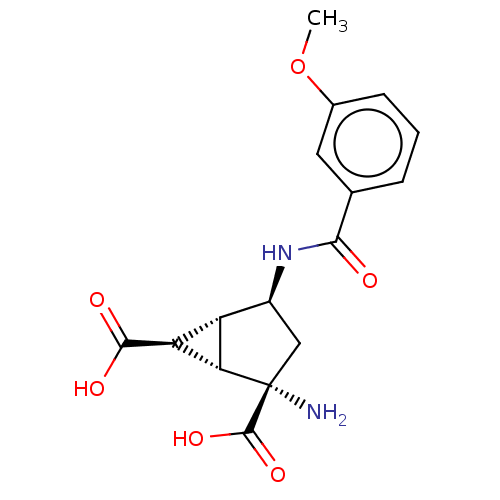
SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@@H]2NC(=O)c1cccc(OC)c1)C(O)=O
InChI Key InChIKey=UXNRHIJPZNNDDJ-VZAVHYRXSA-N
PDB links: 2 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50244219
Found 2 hits for monomerid = 50244219
Affinity DataEC50: >2.50E+4nMAssay Description:Agonist activity at recombinant human mGlu1 receptor expressed in hamster AV12 cells co-expressing rat EAAT1/Galpha15 assessed as induction of increa...More data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+4nMAssay Description:Compound was tested for agonistic activity against D2 receptor from cloned CHO cells, used [3H]U-86170 as radioligandMore data for this Ligand-Target Pair