null
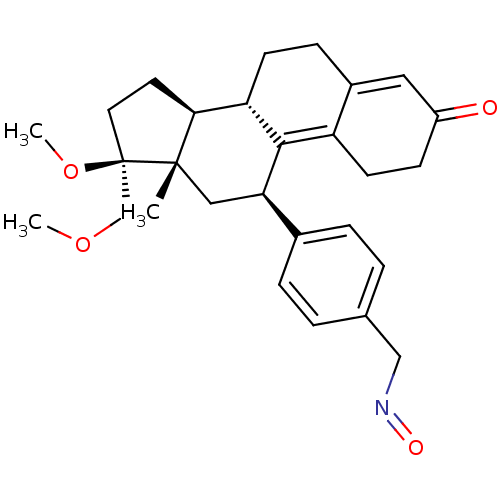
SMILES COC[C@@]1(CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(CN=O)cc1)OC
InChI Key InChIKey=DZKSHTKHHHLSGZ-YTFMROOWSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50375423
Found 2 hits for monomerid = 50375423
Affinity DataIC50: 1.60E+3nMAssay Description:Antagonist activity at human MR ligand binding domain expressed in african green monkey COS7 cells in presence of aldosterone by Gal4 hybrid assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Antagonist activity at mineralocorticoid receptor by cellular reporter gene assayMore data for this Ligand-Target Pair