null
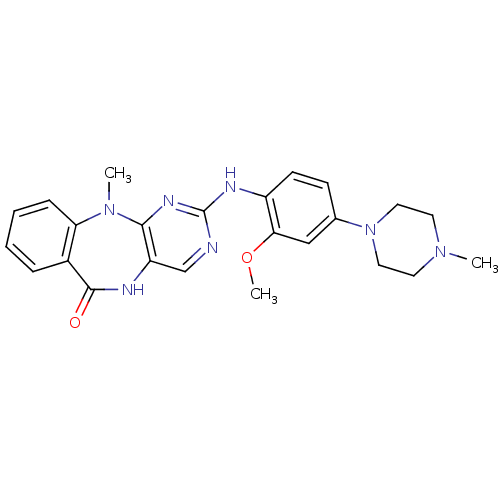
SMILES COc1cc(ccc1Nc1ncc2NC(=O)c3ccccc3N(C)c2n1)N1CCN(C)CC1
InChI Key InChIKey=LGLHCXISMKHLIK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50337126
Found 3 hits for monomerid = 50337126
TargetMitogen-activated protein kinase 7(Homo sapiens (Human))
Dana-Farber Cancer Institute, Inc.
US Patent
Dana-Farber Cancer Institute, Inc.
US Patent
Affinity DataKd: 670nMAssay Description:In vitro Erk5 binding assay: Ambit Kd values in nanomolar. Kd values generated by Ambit binding assay over a concentration range of the compound.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 7(Homo sapiens (Human))
Dana-Farber Cancer Institute, Inc.
US Patent
Dana-Farber Cancer Institute, Inc.
US Patent
Affinity DataKd: >670nMAssay Description:Binding affinity to ERK5 by immobilized ligand displacement assayMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 7(Homo sapiens (Human))
Dana-Farber Cancer Institute, Inc.
US Patent
Dana-Farber Cancer Institute, Inc.
US Patent
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of ERK5 in human HeLa cells assessed as reduction in EGF-induced ERK5 autophosphorylation pretreated for 1 hr followed by EGF stimulation ...More data for this Ligand-Target Pair