null
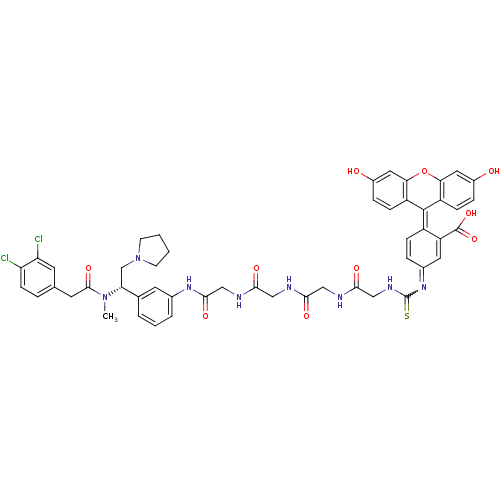
SMILES [#6]-[#7](-[#6@H](-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-1)-c1cccc(-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c1)-[#6](=O)-[#6]-c1ccc(Cl)c(Cl)c1
InChI Key InChIKey=ZFAIYDCFNOMXDL-RRHRGVEJSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50369224
Found 1 hit for monomerid = 50369224
Affinity DataKi: 631nMAssay Description:Binding affinity towards Opioid receptor mu 1 in guinea pig brainMore data for this Ligand-Target Pair