null
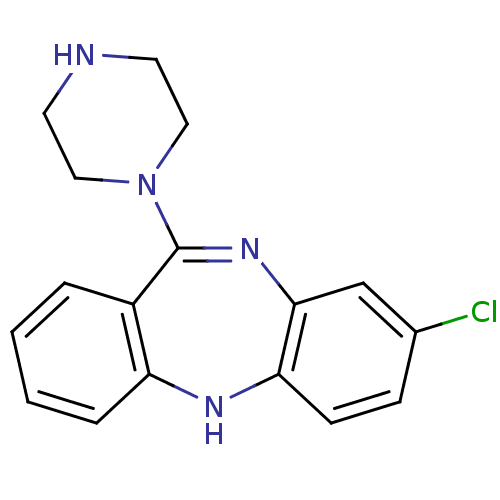
SMILES Clc1ccc2Nc3ccccc3C(=Nc2c1)N1CCNCC1
InChI Key InChIKey=JNNOSTQEZICQQP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50122054
Found 3 hits for monomerid = 50122054
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Universit£ de Strasbourg
Curated by ChEMBL
Universit£ de Strasbourg
Curated by ChEMBL
Affinity DataKi: 60.3nMAssay Description:Displacement of [3H]NMS from EGFP-fused human M1 receptor N-terminal truncated at 17 residues expressed in HEK293 cells after 22 hrs by liquid scinti...More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Universit£ de Strasbourg
Curated by ChEMBL
Universit£ de Strasbourg
Curated by ChEMBL
Affinity DataEC50: 18nMAssay Description:Agonist activity at human muscarinic M1 receptor expressed in CHO-K1 cells assessed as increase of acetylcholine-induced calcium flux by FLIPR assayMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Universit£ de Strasbourg
Curated by ChEMBL
Universit£ de Strasbourg
Curated by ChEMBL
Affinity DataEC50: 48nMAssay Description:Agonist activity at muscarinic M1 receptor (unknown origin) expressed in CHO cellsMore data for this Ligand-Target Pair