null
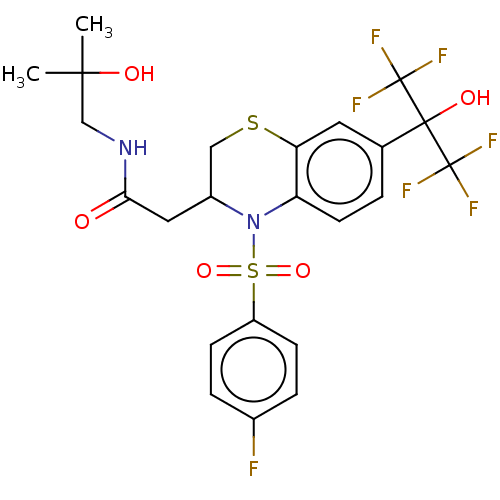
SMILES CC(C)(O)CNC(=O)CC1CSc2cc(ccc2N1S(=O)(=O)c1ccc(F)cc1)C(O)(C(F)(F)F)C(F)(F)F
InChI Key InChIKey=DUBUCROMSYFQOW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 327745
Found 3 hits for monomerid = 327745
Affinity DataEC50: >7.50E+3nMAssay Description:Agonist activity at GAL4-tagged human LXRbeta assessed as receptor activation expressed in African green monkey CV1 cells by luciferase reporter gene...More data for this Ligand-Target Pair
Affinity DataEC50: >7.50E+3nMAssay Description:Agonist activity at GAL4-tagged human LXRbeta assessed as receptor activation expressed in African green monkey CV1 cells by luciferase reporter gene...More data for this Ligand-Target Pair
Affinity DataEC50: 3.71E+3nMAssay Description:Agonist activity at GAL4-tagged human LXRbeta assessed as receptor activation expressed in African green monkey CV1 cells by luciferase reporter gene...More data for this Ligand-Target Pair