null
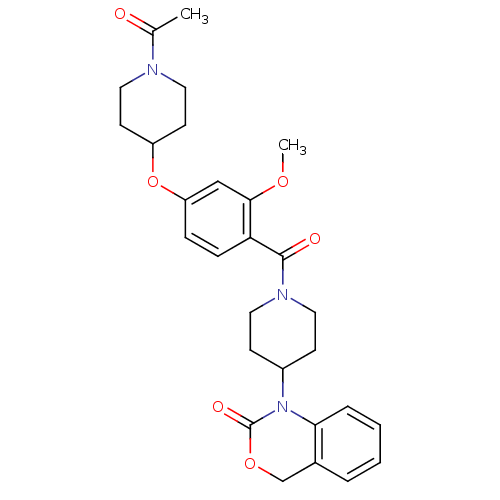
SMILES COc1cc(OC2CCN(CC2)C(C)=O)ccc1C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12
InChI Key InChIKey=WDERJSQJYIJOPD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50029649
Found 14 hits for monomerid = 50029649
Affinity DataKi: 4.60nMAssay Description:Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from humanMore data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:Displacement of [3H]-oxytocin from oxytocin receptor in human uterus tissueMore data for this Ligand-Target Pair
Affinity DataKi: 4.80nMAssay Description:Binding affinity for cloned human oxytocin receptor (OT-R)More data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of 3[H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 9.30nMAssay Description:Binding affinity against cloned human oxytocin receptor from human embryonic kidney cellsMore data for this Ligand-Target Pair
Affinity DataKi: 9.30nMAssay Description:Binding affinity for human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Ability to displace [3H]oxytocin from human OT receptor (hOT)More data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Binding affinity against oxytocin receptor (rOTr) in DES pretreated rat uterineMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Binding affinity for rat uterine oxytocin receptor (rOTr)More data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Binding affinity was evaluated by measuring the displacement of [3H]-OT (oxytocin) from specific binding sites in uterine tissue obtained from ratsMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Displacement of [3H]-oxytocin from oxytocin receptor in rat uterus tissueMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assayMore data for this Ligand-Target Pair
Affinity DataEC50: 4.60nMAssay Description:Activity at human oxytocin receptor expressed in CHO cells by NFAT-luciferase gene reporter assayMore data for this Ligand-Target Pair
Affinity DataIC50: 640nMAssay Description:Compound was tested for displacement of 3[H] oxytocin from rat OT receptor (in vitro)More data for this Ligand-Target Pair