null
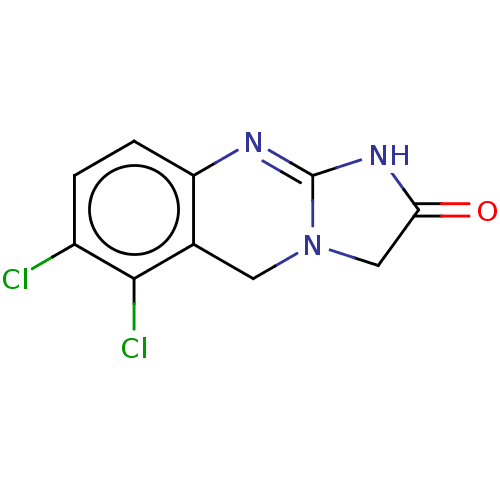
SMILES Clc1ccc2N=C3NC(=O)CN3Cc2c1Cl
InChI Key InChIKey=OTBXOEAOVRKTNQ-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50000334
Found 2 hits for monomerid = 50000334
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 1.05E+3nMAssay Description:Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP)More data for this Ligand-Target Pair
TargetP2Y purinoceptor 12(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 1.05E+3nMAssay Description:Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP)More data for this Ligand-Target Pair