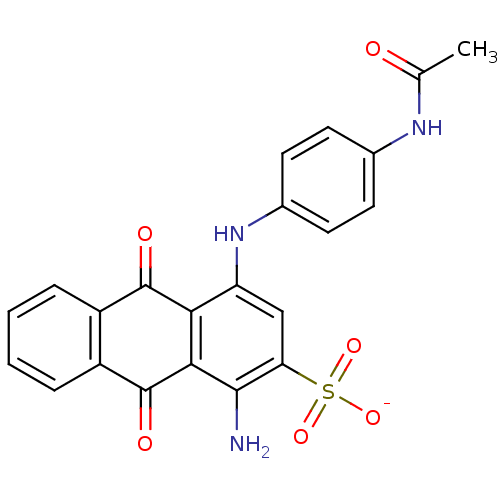
null
SMILES CC(=O)Nc1ccc(Nc2cc(c(N)c3C(=O)c4ccccc4C(=O)c23)S([O-])(=O)=O)cc1
InChI Key InChIKey=CKDSSRXGNPFPPZ-UHFFFAOYSA-M
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50227015
Found 2 hits for monomerid = 50227015
Affinity DataIC50: 5.81E+3nMAssay Description:Antagonist activity at human recombinant P2Y2 receptor in 1321N1 cells assessed as inhibition of UTP-induced calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Antagonist activity at mouse P2Y2 receptor in mouse NG108-15 cells assessed as inhibition of UTP-induced calcium mobilizationMore data for this Ligand-Target Pair