null
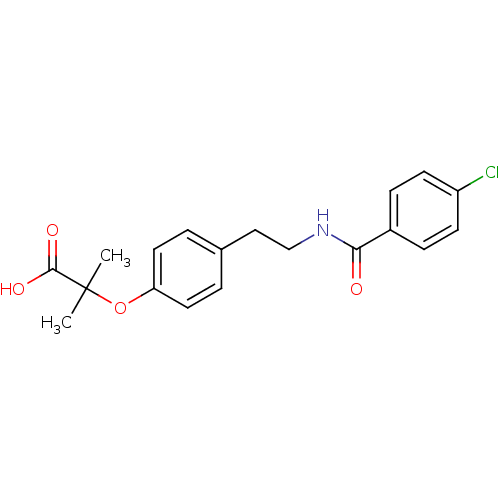
SMILES CC(C)(Oc1ccc(CCNC(=O)c2ccc(Cl)cc2)cc1)C(O)=O
InChI Key InChIKey=IIBYAHWJQTYFKB-UHFFFAOYSA-N
PDB links: 6 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 16 hits for monomerid = 28701
Found 16 hits for monomerid = 28701
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: 5.00E+4nMAssay Description:Agonist activity for Human PPAR alpha receptor in transcriptional activation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Mus musculus)
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: 9.00E+4nMAssay Description:Agonist activity for murine PPAR alpha receptor in transcriptional activation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: 3.57E+4nMAssay Description:In vitro transactivation using receptor transactivation assay against hPPAR alphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: >7.80E+4nMAssay Description:Transactivation of human Peroxisome proliferator activated receptor alpha expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: >7.80E+4nMAssay Description:In vitro effective concentration for agonist activity on human Peroxisome proliferator activated receptor alpha-Gal4 chimeric receptor in transfected...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Mus musculus)
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: 5.00E+4nMAssay Description:Effective concentration against human peroxisome proliferator activated receptor alpha in Gal4 transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: 4.25E+4nMAssay Description:Agonist activity at human PPARalpha expressed in HepG2 cells cotransfected with PPRE3-TK-luc assessed as beta-galactosidase activity after 20 to 22 h...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: 3.00E+4nMAssay Description:Agonist activity at human PPARalpha expressed in monkey CV1 cells by transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: 3.00E+4nMAssay Description:Agonist activity at human PPARalpha expressed in CV1 cells by transactivation assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: 4.66E+4nMAssay Description:Agonist activity at human PPARalpha expressed in HEK293 cells cotransfected with PPREx4-TK-luc assessed as activation of luciferase activity measured...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: 5.00E+4nMAssay Description:Activity at PPARalphaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: 3.17E+4nMAssay Description:Agonist activity at GAL4-tagged PPARalpha-LBD (unknown origin) expressed in HEK293 cells assessed as induction of receptor transactivation incubated ...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Mus musculus)
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: 9.00E+4nMAssay Description:Compound was tested for its agonist activity against murine Peroxisome proliferator activated receptor alpha-Gal4 chimeric receptor transfected CV-1 ...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: 5.00E+4nMAssay Description:Compound was tested for agonist activity on human Peroxisome proliferator activated receptor alpha-Gal4 chimeric receptor in transfected CV-1 cellsMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Glaxo Wellcome Research & Development
Curated by ChEMBL
Glaxo Wellcome Research & Development
Curated by ChEMBL
Affinity DataEC50: 5.00E+4nMAssay Description:The ligand binding domain for PPAR was fused to the yeast transcription factor GAL4 DNA binding domain. CV-1 cells were transiently transfected with ...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)