null
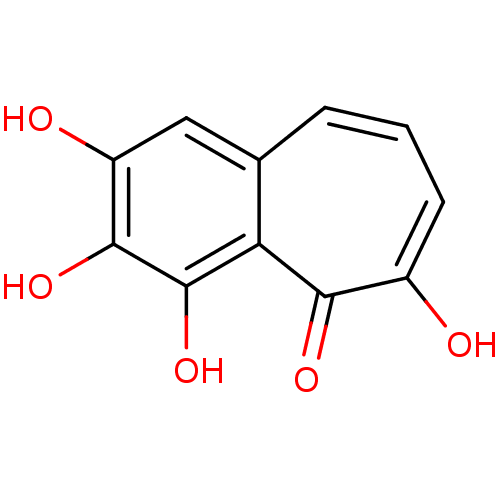
SMILES Oc1cc2cccc(O)c(=O)c2c(O)c1O
InChI Key InChIKey=WDGFFVCWBZVLCE-UHFFFAOYSA-N
PDB links: 4 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50088360
Found 3 hits for monomerid = 50088360
Affinity DataIC50: 7.30E+3nMAssay Description:Inhibition of human SHIP2 catalytic domain (419 to 832 residues) phosphatase activity assessed as inhibition of Ins(1,3,4,5)P4 production using Ins(1...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati...More data for this Ligand-Target Pair
Affinity DataIC50: 8.90E+4nMAssay Description:Inhibition of human SHIP2 catalytic domain (419 to 832 residues) phosphatase activity assessed as phosphate release using Ins(1,3,4,5)P4 as substrate...More data for this Ligand-Target Pair