null
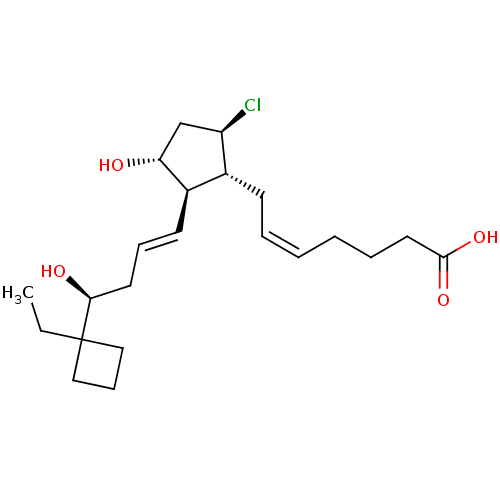
SMILES CCC1(CCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)C[C@@H](Cl)[C@@H]1C\C=C/CCCC(O)=O
InChI Key InChIKey=LBIPUBVVGYRBNA-VGUVCEGPSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50101830
Found 2 hits for monomerid = 50101830
TargetProstacyclin receptor(Homo sapiens (Human))
Emory University School of Medicine
Curated by ChEMBL
Emory University School of Medicine
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Binding affinity to prostanoid IP receptor (unknown origin)More data for this Ligand-Target Pair
TargetProstacyclin receptor(Homo sapiens (Human))
Emory University School of Medicine
Curated by ChEMBL
Emory University School of Medicine
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity towards human Prostanoid IP receptor in CHO cells.More data for this Ligand-Target Pair