null
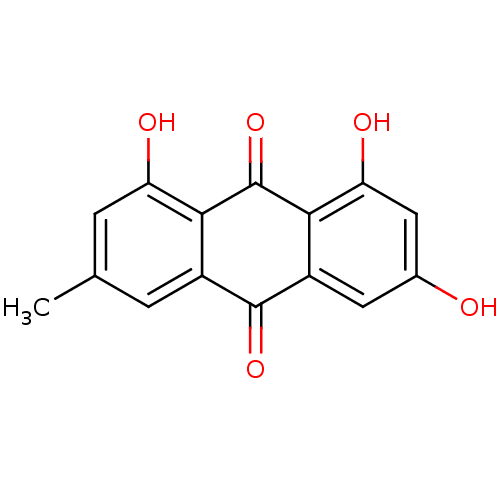
SMILES Cc1cc(O)c2C(=O)c3c(O)cc(O)cc3C(=O)c2c1
InChI Key InChIKey=RHMXXJGYXNZAPX-UHFFFAOYSA-N
PDB links: 14 PDB IDs match this monomer. 2 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 11318
Found 1 hit for monomerid = 11318
TargetProteasome subunit beta type-5(Homo sapiens (Human))
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
Affinity DataIC50: 1.22E+3nMAssay Description:Inhibition of chymotrypsin-like activity of human 26S proteasome assessed as decrease in AMC hydrolysis using Suc-Leu-Leu-Val-Tyr-AMC as substrate in...More data for this Ligand-Target Pair