null
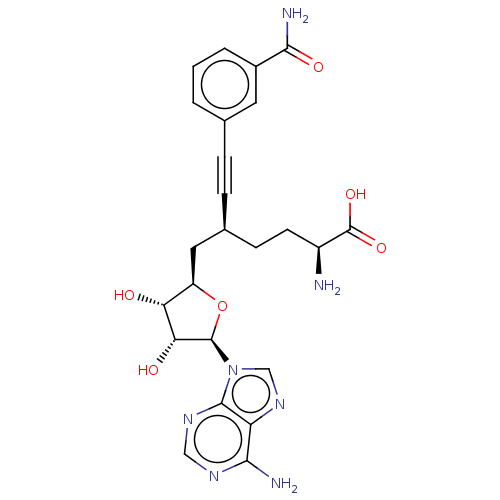
SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O
InChI Key InChIKey=QRKSTGPKAQGBDD-GGPTZFPQSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50530712
Found 2 hits for monomerid = 50530712
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human PRMT1 expressed in Escherichia coli assessed as reduction in methylated histone H4 full length level using histone H4 full length...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human PRMT1 expressed in Escherichia coli assessed as reduction in methylated histone H4 full length level using histone H4 full length...More data for this Ligand-Target Pair