null
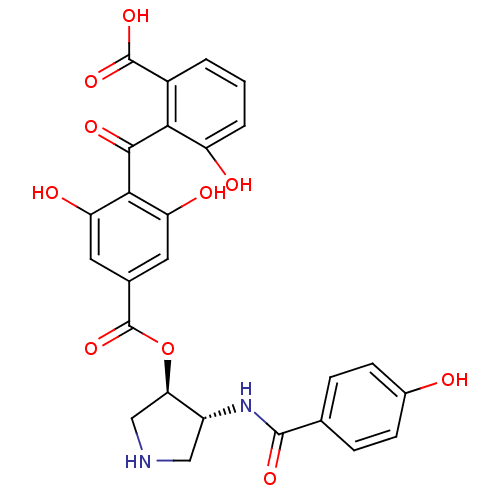
SMILES OC(=O)c1cccc(O)c1C(=O)c1c(O)cc(cc1O)C(=O)O[C@@H]1CNC[C@H]1NC(=O)c1ccc(O)cc1
InChI Key InChIKey=YIZRPXUMLQCIMQ-OXQOHEQNSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 3152
Found 14 hits for monomerid = 3152
Affinity DataIC50: 10nMAssay Description:Inhibition of protein kinase C beta 1More data for this Ligand-Target Pair
Affinity DataIC50: 33nMpH: 7.5 T: 2°CAssay Description:PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs.More data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs.More data for this Ligand-Target Pair
Affinity DataIC50: 33nMpH: 7.5 T: 2°CAssay Description:The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his...More data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Inhibition of protein kinase C beta IIMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C beta 1 isozymeMore data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C beta 2 isozymeMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of Protein kinase C beta 1More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of protein kinase C beta IMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of human Protein kinase C beta 1More data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Inhibition of Protein kinase C beta 2More data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Inhibition of human Protein kinase C beta 2More data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Inhibition of protein kinase C epsilonMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMpH: 7.5 T: 2°CAssay Description:PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs.More data for this Ligand-Target Pair