null
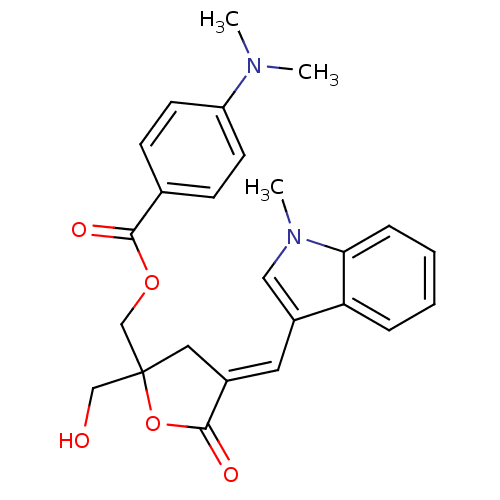
SMILES CN(C)c1ccc(cc1)C(=O)OCC1(CO)C\C(=C/c2cn(C)c3ccccc23)C(=O)O1
InChI Key InChIKey=OTCSXQIUVAXJNR-LDADJPATSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50244974
Found 2 hits for monomerid = 50244974
TargetRas guanyl-releasing protein 3(Homo sapiens (Human))
National Cancer Institute at Frederick
Curated by ChEMBL
National Cancer Institute at Frederick
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Displacement of [3H]PDBu from RasGRP3 (unknown origin) expressed in cellsMore data for this Ligand-Target Pair
TargetRas guanyl-releasing protein 3(Homo sapiens (Human))
National Cancer Institute at Frederick
Curated by ChEMBL
National Cancer Institute at Frederick
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation countingMore data for this Ligand-Target Pair