null
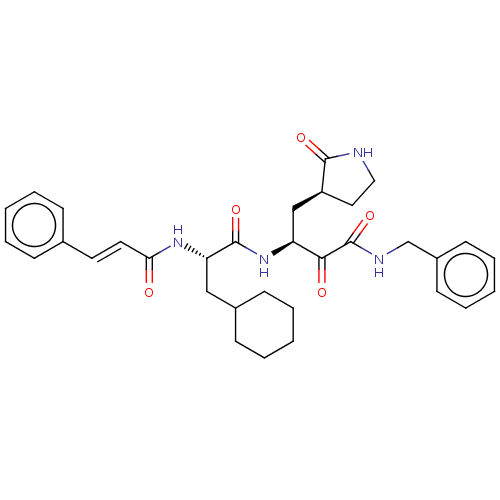
SMILES O=C(N[C@@H](CC1CCCCC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)C(=O)NCc1ccccc1)\C=C\c1ccccc1
InChI Key InChIKey=QTGORSKTHIBLJT-FIVJMQNTSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 420283
Found 1 hit for monomerid = 420283
TargetReplicase polyprotein 1ab [3264-3569](Severe acute respiratory syndrome coronavirus 2)
University of Lubeck
University of Lubeck
Affinity DataIC50: 180nMAssay Description:A fluorescent substrate harboring the cleavage site (indicated by the arrow, ↓) of SARS-CoV-2 Mpro(Dabcyl-KTSAVLQ↓SGFRKM-E(Edans)-NH2; GL...More data for this Ligand-Target Pair
Ligand Info