null
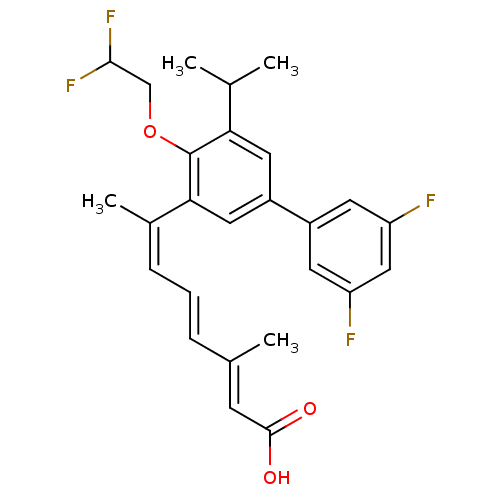
SMILES CC(C)c1cc(cc(\C(C)=C/C=C/C(/C)=C/C(O)=O)c1OCC(F)F)-c1cc(F)cc(F)c1
InChI Key InChIKey=WPVHQRRTUJTHOM-HSQINHPTSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50135461
Found 2 hits for monomerid = 50135461
TargetRetinoic acid receptor RXR-alpha(Homo sapiens (Human))
Ligand Pharmaceuticals Inc
Curated by ChEMBL
Ligand Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 2.60nMAssay Description:Binding affinity against RXR alpha receptor using [3H]-9-cis-RA as radioligand in CV-1 cellsMore data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha(Homo sapiens (Human))
Ligand Pharmaceuticals Inc
Curated by ChEMBL
Ligand Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataIC50: 4.60nMAssay Description:In vitro agonistic activity against PPAR gamma along with 100 nM BRL49653More data for this Ligand-Target Pair