null
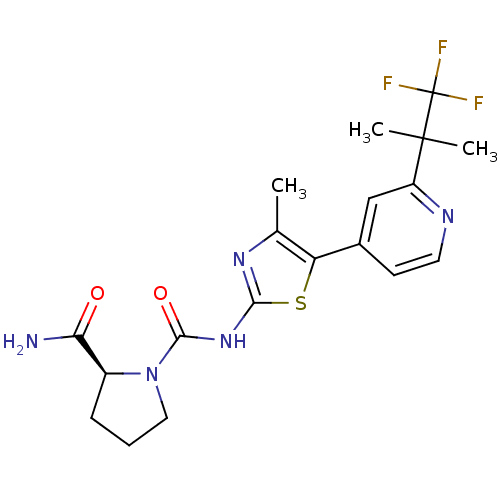
SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F
InChI Key InChIKey=STUWGJZDJHPWGZ-LBPRGKRZSA-N
PDB links: 8 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50436459
Found 3 hits for monomerid = 50436459
TargetSerine/threonine-protein kinase mTOR(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 9.10E+3nMAssay Description:Inhibition of mTOR (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase mTOR(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.46E+3nMAssay Description:Inhibition of N-terminal FLAG-tagged human recombinant mTOR (1362 to end residues) using ULight-4E-BP1 (Thr37/46) peptide as substrate incubated for ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase mTOR(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of mTOR in TSC1 null human MCF7 cells assessed as decrease in p70S6K phosphorylation at Thr389 residue incubated for 2 hrs by ELISAMore data for this Ligand-Target Pair